



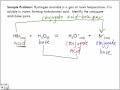














Conjugate acids and bases are part of the Bronsted-Lowry theory of acids and bases. According to this theory, the species that donates a hydrogen cation or proton in a reaction is a conjugate acid, while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base. The conjugate base may be recognized as an anion. COMEDK 2012: The conjugate acid of NH2- is (A) N2H4 (B) NH4+ (C) NH2OH (D) NH3 . Check Answer and Solution for above question from Chemistry Anwendungsbeispiele für “conjugate acid” in einem Satz aus den Cambridge Dictionary Labs Examples of how to use “conjugate acid” in a sentence from the Cambridge Dictionary Labs In other words, a conjugate acid is the acid member, HX, of a pair of compounds that differ from each other by gain or loss of a proton. A conjugate acid can release or donate a proton. A conjugate base is the name given to the species that remains after the acid has donated its proton. The conjugate base can accept a proton. Acid strength is determined by the amount of that acid that actually ionizes. Acids are molecular covalent compounds which you don't expect to ionize (release an #H^+# and leave behind the conjugate base, or #Cl^-# for example).. The strongest acids ionize 100%. There are 6 that most consider to be the "STRONG" acids: HCl, HI, HBr, HNO_3 #, H_2SO_4# and HClO_4#. dict.cc | Übersetzungen für 'conjugate acid' im Isländisch-Deutsch-Wörterbuch, mit echten Sprachaufnahmen, Illustrationen, Beugungsformen, A conjugate acid is the particle produced when a base accepts a proton. The hydrogen sulfate ion is the conjugate acid of the sulfate ion. A conjugate base is the particle produced when an acid donates a proton. When acting as a base and reacting with water, Ammonia NH 3 can form a conjugate acid when it forms ammonium NH 4 +. In the reaction illustrated below, water serves both as acid and Conjugate Acid-Base Pairs. Acids and bases exist as conjugate acid-base pairs.The term conjugate comes from the Latin stems meaning "joined together" and refers to things that are joined, particularly in pairs, such as Brnsted acids and bases.. Every time a Brnsted acid acts as an H +-ion donor, it forms a conjugate base.Imagine a generic acid, HA. When this acid donates an H + ion to water Conjugate Acid-Base Pair: Consider the weak monoprotic acid {eq}HA{/eq}. When this acid donates its proton, it becomes a weak base, {eq}A^-{/eq}. {eq}HA{/eq} and {eq}A^-{/eq}, are called conjugate
[index] [3501] [3891] [7526] [3058] [5327] [530] [3548] [9115] [2181] [4068]
In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... Watch more videos on http://www.brightstorm.com/science/chemistrySUBSCRIBE FOR All OUR VIDEOS!https://www.youtube.com/subscription_center?add_user=brightstor... All this talk of conjugate sounds scary! Not really, this video looks at what a conjugate base and acid are with multicoloured equations. Take a look to find... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Conjugate acid-base pairing is a concept which derives from the Broasted-Lowry Theory. In this section of Module 6: Acids and Bases in the HSC Chemistry Syll... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Donate here: http://www.aklectures.com/donate.php Website video: http://www.aklectures.com/lecture/conjugate-acid-base-pairs Facebook link: https://www.faceb...
Copyright © 2024 m.playrealmoneygames.xyz